In clinical trials, it’s not always possible to measure hard endpoints like cardiovascular disease events and cancer remission rates. Studies that use clinical outcomes often dichotomize these variables, and as a result, they need to have a large number of participants and be long in duration to detect differences between groups.
Again, this type of research is expensive and not always feasible. In many scenarios, a more practical alternative is to focus on intermediate markers. Intermediate markers are biomarkers associated with a clinical outcome.hulley2007?
For example, C-reactive protein (CRP) is a molecule that is strongly associated with inflammation, and you can often find that CRP levels increase when inflammation increases.bray2016w?,chew2012jets?,pepys2003jci? Because inflammation is associated with coronary heart disease (CHD)Frangogiannis2012-fo?,Hansson2005-wz?,Libby2006-ch?,Ruparelia2017-nf?, an investigator may choose to focus on decreasing levels of inflammation in a clinical trial (measured by CRP), rather than focus on how many CHD-related deaths the intervention prevents.
If the changes in an intermediate marker can robustly predict changes in a hard endpoint, and if it’s part of the primary pathway of the clinical outcome, then your biomarker can be considered a surrogate marker for that clinical outcome.hulley2007? (I’m not sure if CRP is a good surrogate outcome for CHD events, just used it as an example).
Low-density lipoprotein (LDL) is considered an excellent surrogate marker for CHD because reducing LDL levels also seems to reduce the number of CHD events.Cholesterol_Treatment_Trialists_CTT_Collaboration2015-ni?,Taylor2013-te? Unfortunately, this can all go wrong if the intermediate marker is associated with a clinical outcome, but is not involved in the causal pathway of the outcome and is confounded by other phenomena.
Torcetrapib
A great example of this is the story of high-density lipoprotein (HDL) and myocardial infarction (heart attacks). Several studies had found associations between low levels of HDL, often considered “good cholesterol,” and heart attacks.Assmann1996-jd?,Curb2004-ee?,Gordon1977-le?,Gordon1989-mc?,Rahilly-Tierney2011-ox?,Sharrett2001-su?,Turner1998-gv?
So, it shouldn’t come as a surprise that a drug (torcetrapib) was produced by Pfizer that attempted to increase the amount of HDL with the hopes that it would reduce the number of CVD events.
A group of researchers administered the drug to thousands of patients.1 The drug was successful in changing the lipids of the participants to more favorable numbers. Patients who received the drug had a 24.9% decrease in LDL and a 72.1% increase in HDL. Seems pretty great. However, the number of deaths increased by 58% and the number of heart attacks increased by 21%.
A systematic review and meta-analysisBriel2009-yo? published in the BMJ a few years later concluded the following after pooling studies that focused on interventions that primarily increased HDL and interventions that primarily decreased LDL,
“Available data suggest that simply increasing the amount of circulating high-density lipoprotein cholesterol does not reduce the risk of coronary heart disease events, coronary heart disease deaths, or total deaths. The results support reduction in low-density lipoprotein cholesterol as the primary goal for lipid modifying interventions.”
SvenssonSvensson2013-vq? provides us with a very lovely table showing other scenarios for which a drug had a favorable effect on a surrogate marker but had a negative impact on the clinical outcome.
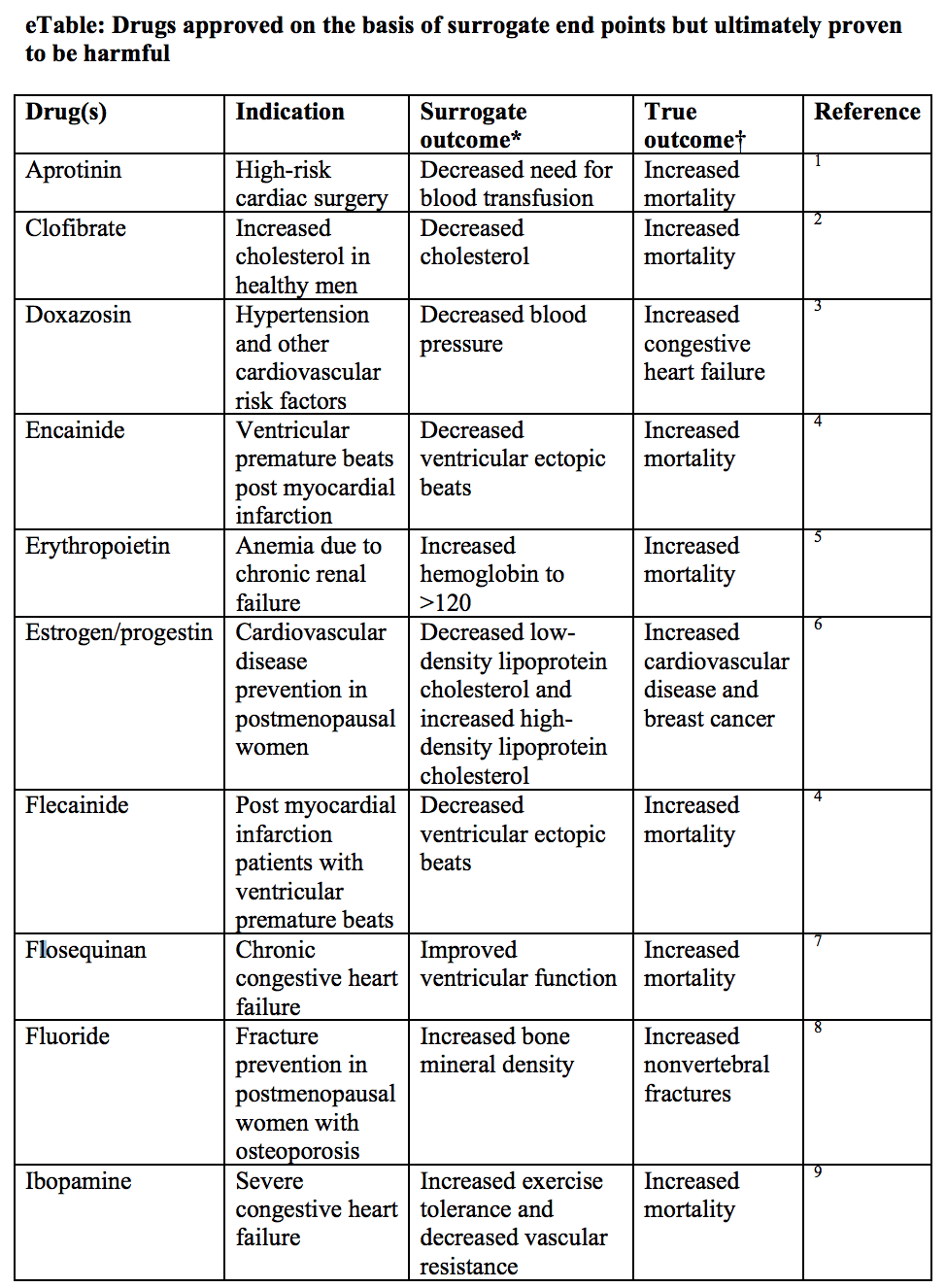
What does this tell us? That focusing only on improving surrogate markers is not a very good idea if there is not much concordance between trials that focus on surrogates and trials that focus on hard endpoints. Until we’ve established a robust causal link between a surrogate marker and a hard endpoint, we shouldn’t be seduced by surrogates.
References
Help support the website!


